Single-step synthesis of novel chloroaluminate ionic liquid for green Friedel–Crafts alkylation reaction
Mangesh Sakhalkar1,2, Pavankumar Aduri1, Sharad Lande1, Sudeshna Chandra2*
Clean Technologies and Environmental Policy, 2019, pp 1-13 doi:10.1007/s10098-019-01769-y (Impact Factor: 2.277)
1Reliance Industries Limited, Mumbai, India
2Sunandan Divatia School of Science, SVKM’S NMIMS, Mumbai 400056, India
*Corresponding author
Abstract
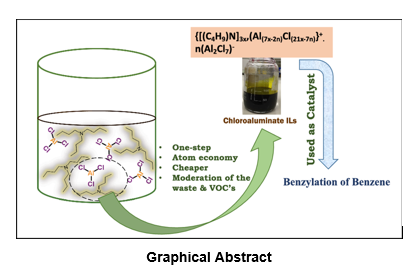
Friedel–Crafts alkylation reactions constitute a very important class of reactions which are usually catalysed in the liquid phase using Lewis acids at the industrial scale. Diphenyl methane synthesised by Friedel–Crafts alkylation from benzene with benzyl chloride has been interesting in organic synthesis, perfumes and dyes. The new environmental legislation recommends a green and reusable catalyst system that is environmentally benign which leads to minimal pollution and waste generation. Chloroaluminate ionic liquids (ILs) have been used immensely as homogeneous catalyst in the organic synthetic processes. Though these ILs have multiple advantages, their synthesis process involves certain disadvantages like multiple step synthesis, viz. use of solvents and its removal after reaction, removal of precursors, solvent washing for removal of trace impurities and drying of salt/adduct. We have established a novel single-step synthesis method of chloroaluminates from tributylamine and aluminium chloride via amine–aluminium chloride adduction using suitable solvent or one of the reactants as reaction medium. The process improved the atom economy, reduced the use of volatile organic chemicals and drastically minimised the generation of wastes. Formation of chloroaluminate ILs was confirmed by FTIR, 27Al NMR and mass spectroscopy (ESI–MS), and other physicochemical properties were also determined. The chloroaluminate IL was used as catalyst for Friedel–Crafts benzylation of benzene. The performance of the ILs as catalyst showed higher catalytic activity and could be recycled twice for benzylation reaction. Reaction parameters were also optimised to achieve higher yields of diphenyl methane. Tributylamine can be easily recovered by hydrolysis of spent IL which would moderate the chemical oxygen demand in generated effluent.
Online Link of the paper - http://link.springer.com/article/10.1007/s10098-019-01769-y